Experiment #7 Supplemental Information 实验 #7 补充信息
Content from Beran: 来自 Beran 的内容:
- Galvanic Cells; the Nernst Equation (Experiment 32 in Beran - pages 357-368)
- We will ONLY be doing Parts A and B.
- Electrolytic Cells; Avogadro’s Number (Experiment 33 in Beran - pages 369 - 376)
- We will ONLY be doing Part A.
32.A B
电化学电池;能斯特方程(Beran 中的实验 32 - 第 357-368 页)
我们将只做 A 部分 和 B 部分。
33.A
电解池;阿伏伽德罗常数(Beran 中的实验 33 - 第 369 - 376 页)
我们将只做 A 部分。
You need to write a prelab in your Lab Archives electronic laboratory notebook (see description in the Additional Course Information pdf available on Courseworks). The prelab consists of a purpose, materials/instrumentation/safety considerations, procedure, and data tables. Your procedure should be written in your own words and include all the steps of the experiment.
你需要在你的 Lab Archives 电子实验室笔记本 中写一份 预实验报告(参见 Courseworks 上提供的 附加课程信息 pdf 中的 描述)。预实验报告 包括 目的、材料/仪器/安全注意事项、步骤 和 数据表。你的 步骤 应该用你自己的 语言 写,并包括 实验 的所有 步骤。
During your lab session, you are expected to be in adherence to the dress code that is outlined in the syllabus. You will be expected to be wearing your lab coat and protective eyewear during lab. During experimentation, you are expected to be wearing gloves. If something spills or breaks, please speak to a TA or the Instructor immediately.
在你的 实验课 期间,你需要遵守 教学大纲 中概述的 着装规范。你需要在 实验课 期间穿 实验服 和防护 眼镜。在 实验 期间,你需要戴 手套。如果有什么东西溢出或破损,请立即与 助教 或 指导老师 交谈。
Additional Resources: 附加资源:
The following excerpts can be found in Chemical Principles by Steven S. Zumdahl and Donald J. DeCoste. References are provided for both the Edition (2013) and Edition (2017). They are not required reading, but are provided as an extra resource if you need help understanding the concepts covered in this experiment or would like to learn more about the concepts in this experiment.
以下 摘录 可以在 Steven S. Zumdahl 和 Donald J. DeCoste 的 化学原理 中找到。为第 版 (2013) 和第 版 (2017) 都提供了 参考。它们不是必读的 内容,但如果你需要 帮助 理解本 实验 中涵盖的 概念,或者想了解更多关于本 实验 中的 概念,它们可以作为额外的 资源 提供。
- Chapter 11, pages ( Edition); 398-417 ( Edition)
- Chapter 11, pages 502-510 ( Edition); 425-433 ( Edition)
章节 11,第 474 - 492 页(第 版);398-417(第 版)
- 章节 11,第 502-510 页(第 版);425-433(第 版)
●Changes to the Experiment and Tips: 实验的更改和提示:
32.A Galvanic Cells; the Nernst Equation, Part A: Reduction Potentials of Several Redox Couples 电池;能斯特方程,A 部分:几种氧化还原对的还原电位
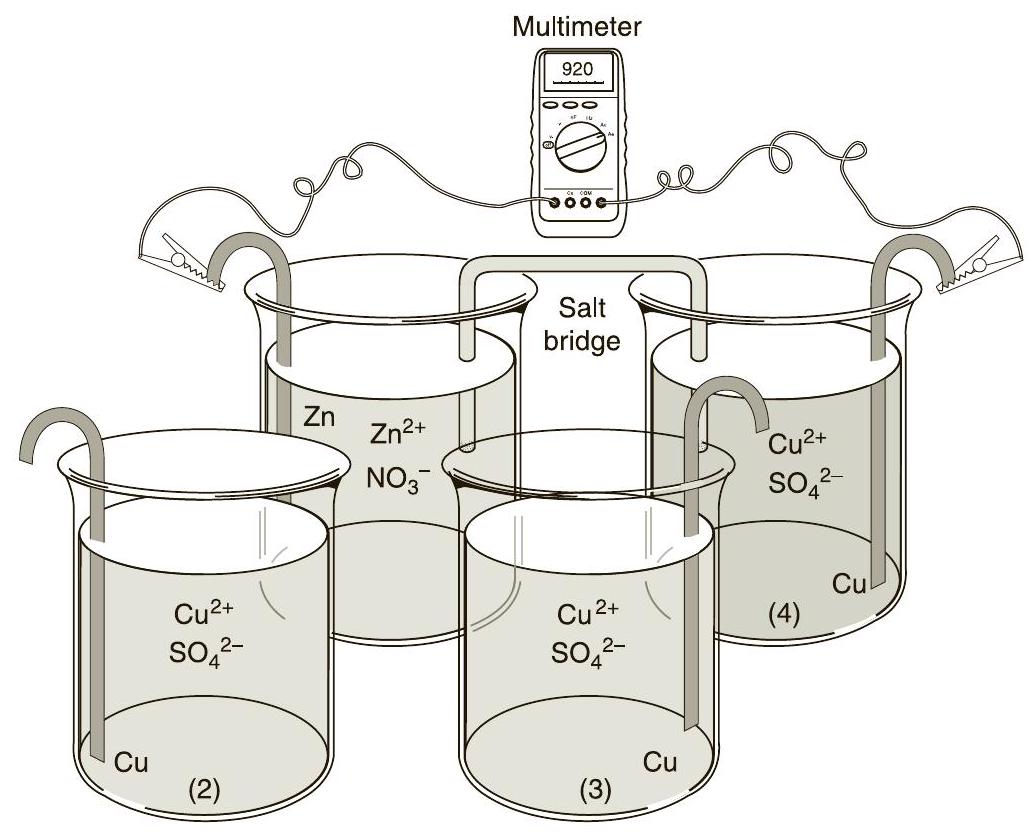
Figure 32.3 Setup for measuring the cell potentials of sixgalvanic cells
图 32.3 六个电池电势测量的装置
Step #1: Collect the Electrodes, Solutions, and Equipment 步骤 #1:收集电极、溶液和设备
Obtain four large glass test tubes and fill them three-fourths full of the solutions as shown in Figure 32.3. Share these solutions with other chemists/groups of chemists in the laboratory.
获取四个的玻璃试管,并如图 32.3 所示,将它们装满四分之三的 溶液。与实验室中的其他化学家/化学家组共享这些溶液。
you will be using large glass test tubes. They are not in the drawers, but will be provided to you at the start of the lab session. They will be pre-filled for you. You will use the same set of test tubes for all of Part A (four test tubes total).
用四个大的玻璃试管装溶液。它们不在抽屉里,但在实验课开始时会提供给您。它们会预先为您填充。对于A部分,您将使用相同的一套试管(总共四个试管)。
Polish strips of copper, zinc, magnesium, and iron metal with sandpaper, and rinse with deionized water. These polished metals, used as electrodes.
用砂纸打磨铜、锌、镁和铁金属的条带,然后用去离子水冲洗。这些抛光的金属用作电极。
make sure to thoroughly polish the electrodes with sandpaper and rinse them with deionized water. Use a Kim-wipe to dry them before use.
请确保用砂纸彻底抛光电极,并用去离子水冲洗。使用金佰利纸巾在使用前擦干它们。
Do not bend the electrodes over the sides of the test tubes. You will dip the electrodes into the appropriate solutions to complete the cell for each trial.
不要将电极弯曲到试管的侧面。您将把电极浸入适当的溶液中,以完成每次试验的电池。
Check out a multimeter (Figure 32.4) with two electrical wires (a red and black wire) attached to alligator clips.
检查一个带有两根电线(红色和黑色电线)连接到鳄鱼夹的万用表(图 32.4)
Obtain a multimeter, one red wire with an alligator clip, and one black wire with an alligator clip. If the alligator clips seem rusted, polish them with sandpaper and rinse them with deionized water. Dry them with a Kim-wipe before using them.
获得一个万用表,一根带有鳄鱼夹的红色电线和一根带有鳄鱼夹的黑色电线。如果鳄鱼夹看起来生锈了,用砂纸抛光它们,并用去离子水冲洗。在使用前用金佰利纸巾擦干它们。
use the "red, right, plus" rule in connecting the red wire of the multimeter to the right-side positive electrode (cathode) of the galvanic cell
使用“红、右、加”规则将万用表的红色导线连接到原电池的右侧正电极(阴极)
Step #2: Set Up the Copper/Zinc Cell 步骤 #2:设置铜/锌电池
Attach one alligator clip to the copper electrode and the other alligator clip to the zinc electrode. Do not put the electrodes into the solutions yet!
将一个鳄鱼夹红色连接到右侧的铜Cu电极,将另一个鳄鱼夹黑色连接到左侧的锌Zn电极。不要将电极放入溶液中!
右侧=铜Cu=红色=电势高阴极Cathode=+
左侧=锌Zn=黑色=电势低阳极Aanode=-
Obtain a long, narrow strip of filter paper (at the front of lab) and saturate it in solution. Using two glass stir-rods, carefully place one end of the wet filter paper in the copper solution and the other end in the zinc solution (this is the salt bridge). Make sure that the filter paper adheres to the sides of the test tubes.
取一条长的、窄的滤纸条(在实验室的前面),并将其浸泡在 溶液中。
使用两个玻璃搅拌棒,小心地将湿滤纸的一端放入铜溶液中,另一端放入锌溶液中(这是盐桥)。
确保滤纸粘附在试管的侧面。
Set the multimeter to the " 2 V " setting.
将万用表设置为“2 V”设置。
Step #3: Determine the Copper/Zinc Cell Potential 步骤 #3:确定铜/锌电池电位
Simultaneously, dip the zinc electrode into the zinc solution and the copper electrode into the copper solution. A reading should register on the multimeter. (you need to record positive values).
同时,将锌电极=左侧黑阳极anode浸入锌溶液中,并将铜电极=右侧红阴极cathode浸入铜溶液中。
万用表上应显示正读数。(您需要记录正值)。
Determine the copper-zinc cell potential. If the multimeter reads a negative potential, reverse the connections to the electrodes.
确定 铜锌电池电位 。如果多用表显示负的 电位 ,则反转电极的 连接 。
Read and record the (positive) cell potential.
读取并记录(正) 电池电位 。
Record the first stable reading on the multimeter (stable means it should remain on that number for at least three seconds).
在万用表上记录第一个稳定的读数(稳定意味着它应在该数字上保持至少三秒)。
Identify the metal strips that serve as the cathode (positive terminal) and the anode.
Write an equation for the half-reaction occurring at each electrode.
Combine the two half-reactions to write the equation for the cell reaction.
识别作为 阴极 (正端子)和阳极的 金属条 。
写出在每个电极上发生的半反应的方程式。
将两个半反应结合,写出电池反应的方程式。
From the positive reading of the multimeter, you will calculate what species is being oxidized and what species is being reduced. The electrode attached to the positive ( + ) terminal (red port) of the multimeter is where reduction occurs and that half reaction has the more positive reduction potential. Record which metal is being reduced and which one is being oxidized in your lab notebook.
根据万用表的正读数,您将计算出什么物质被氧化↑,什么物质被还原↓。
连接到万用表的正(+)端子(红色端口)的电极是发生还原反应↓的地方,并且该半反应具有更高的正还原电位E。
在您的实验笔记本中记录哪种金属被还原↓=红右加阴C,哪种被氧化↑=黑左负阳A。
Step #4: Repeat for the Remaining Cells 步骤 #4:重复剩余的电池
Determine the cell potentials for the cells listed on the Report Sheet (Beran page 365, Part A). Follow the same procedure that was done above for the copper/zinc cell. Use a new salt bridge for each cell. Remember to polish and rinse the electrodes for every trial.
确定报告单(Beran第365页,A部分)上列出的电池的电池电位。
按照与上述铜/锌电池相同的程序进行操作。
为每个电池使用新的盐桥。
记住每次试验都要抛光和冲洗电极。
| Galvanic Cell | E_cell Measured | Anode ⊖Black Left | Equation for Anode Half-Reaction | Cathode ⊕Red Right | Equation for Cathode Half-Reaction |
|---|---|---|---|---|---|
| Cu-Zn | Zn | Zn(s) → Zn²⁺(aq) + 2e⁻ | Cu | Cu²⁺(aq) + 2e⁻ → Cu(s) | |
| Cu-Mg | Mg | Mg(s) → Mg²⁺(aq) + 2e⁻ | Cu | Cu²⁺(aq) + 2e⁻ → Cu(s) | |
| Cu-Fe | Fe | Fe(s) → Fe²⁺(aq) + 2e⁻ | Cu | Cu²⁺(aq) + 2e⁻ → Cu(s) | |
| Zn-Mg | Mg | Mg(s) → Mg²⁺(aq) + 2e⁻ | Zn | Zn²⁺(aq) + 2e⁻ → Zn(s) | |
| Fe-Mg | Mg | Mg(s) → Mg²⁺(aq) + 2e⁻ | Fe | Fe²⁺(aq) + 2e⁻ → Fe(s) | |
| Zn-Fe | Zn | Zn(s) → Zn²⁺(aq) + 2e⁻ | Fe | Fe²⁺(aq) + 2e⁻ → Fe(s) |
Step #5: Determine the Relative Reduction Potentials 步骤 #5:确定相对还原电位
Determine the relative reduction potentials. Assuming the reduction potential of the redox couple is -0.79 V , calculate the reduction potentials of all other redox couples.
确定相对的还原电位。假设 氧化还原对的还原电位为 -0.79 V ,计算所有其他氧化还原对的还原电位。
These calculations will be done for the lab report. They do not need to be included on LabArchives.
这些计算将为实验报告完成。它们不需要包含在LabArchives上。
32.B Galvanic Cells; the Nernst Equation, Part B: Effect of Concentration Changes on Cell Potential 电池;能斯特方程,B 部分:浓度变化对电池电位的影响
Step #1: Effect of Different Molar Concentrations 步骤 #1:不同摩尔浓度的影响
Obtain two new test tubes - one with and one with .
获得两个新的试管 - 一个装有 ,一个装有 。
Set up a galvanic cell as in Part A under the canopy hood, but use the new set of test tubes. You will need a new salt bridge and two Cu electrodes. Measure the positive potential of the cell and determine the species being oxidized and reduced as in Part A.
像A部分一样,在通风橱下建立一个原电池,但使用新的一套试管。
您将需要一个新的盐桥和两个铜电极。
测量电池的正电位,并确定如A部分中被氧化和还原的物质。
Immerse a polished copper electrode in each solution. Prepare a salt bridge (Part A.2) to connect the two half-cells. Measure the cell potential. Determine the anode and the cathode. Write an equation for the reaction occurring at each electrode.
将抛光的铜电极浸入每个溶液中。准备一个盐桥(A.2 部分)以连接两个半电池。测量电池电势。确定阳极和阴极。为每个电极上发生的反应编写一个方程式。
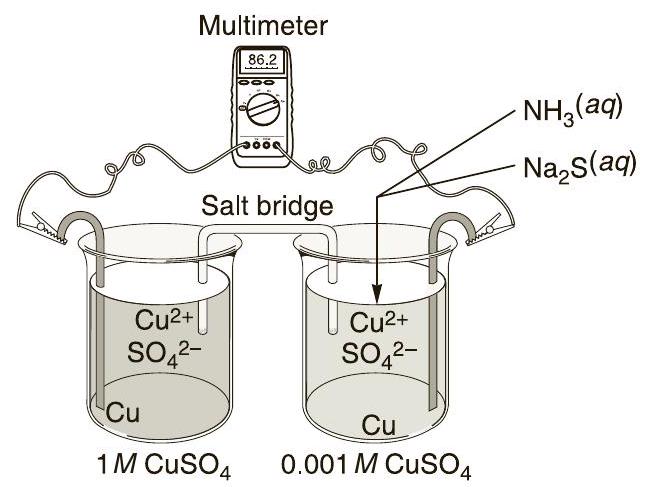
Figure 32.5 Setup for measuring the cell potential of a concentration cell
| Step 1 | Anode ⊖Black Left | Cathode⊕Red Right |
|---|---|---|
| 0.001 M CuSO₄ + Cu | 1 M CuSO₄ + Cu | |
| Cell potential E of concentration cell (V) | ||
| Anode half-reaction: | Cu(s) → Cu²⁺(0.001M) + 2e⁻ | |
| Cathode half-reaction: | Cu²⁺(1M) + 2e⁻ → Cu(s) | |
| Explain why a potential is recorded. |
Step #2: Effect of Complex Formation 步骤 #2:络合效应
Use a 50 mL beaker to obtain about 10 mL of ammonia (bring the stock bottle to your lab bench and pour out under your hood; keep the beaker under the canopy hood due to the fumes).
使用一个50毫升的烧杯取大约10毫升 氨水 (将储液瓶带到实验台,且在通风橱下倒出;由于 气味 ,保持烧杯在排气罩下)。
You can use a plastic transfer pipette to add the ammonia to the test tube.
你可以使用塑料移液管将氨水转移到试管中。
Effect of complex formation. Add of 6M to the solution until any precipitate redissolves.
向 溶液中加入 的6M ,直到所有沉淀物重新溶解。
(Caution: Do not inhale .)
(注意:不要吸入 。)
Make sure to mix the solution in the test tube to ensure homogeneity.
Do not take the potentil reading until after adding the ammonia and ensuring homogeneity.
确保充分混合试管中的溶液以保证均匀性。
在加入氨水并确保溶液均匀之前,不要进行电位测量。
Then, record the cell potential as previously done and make the appropriate observations.
然后,按照之前的步骤记录电池电位并做出相应的 观察 。
Observe and record any changes in the half-cell and the cell potential.
观察并记录半电池和电池电势中的任何变化。
| Step 2 | |
|---|---|
| Cell potential from complex formation (V) | |
| Observation of solution in half-cell. | |
| Explain why the potential changes as it does with the addition of NH₃(aq). |
Step #3: Effect of Precipitate Formation 步骤#3:沉淀形成的影响
Use a 50 mL beaker to obtain about 10 mL of sodium sulfide (bring the stock bottle to your lab bench and pour out under your hood; keep the beaker under the canopy hood due to the pungent smell).
使用 50 mL 的烧杯获取约 10 mL 的硫化钠NaS(将储备瓶带到您的实验台上,并在通风橱下倒出;由于刺鼻的气味,请将烧杯放在通风橱下)。
You can use a plastic transfer pipette to add the sodium sulfide to the test tube.
Add of to the 0.001 M solution now containing the added .
Make sure to mix the solution in the test tube to ensure homogeneity.
您可以使用塑料转移吸管将硫化钠添加到试管中。
向现在包含添加的 的 0.001 M 溶液中加入 的 。
确保混合试管中的溶液以确保均匀性。
Do not take the potential reading until after adding the sodium sulfide and ensuring homogeneity. You most likely will not see a precipitate, but record whatever you see. Then, record the cell potential as previously done.
在添加硫化钠并确保均匀性后才能进行电势读数。
您很可能看不到沉淀,但请记录您所看到的任何内容。
然后,记录之前完成的电池电势。
What is observed in the half-cell and what happens to the cell potential? Record your observations.?
在半电池中观察到什么,电池电势会发生什么?记录你的观察。
| Step 3 | |
|---|---|
| Cell potential from precipitate formation: | |
| Observation of solution in half-cell. | |
| Explain why the potential changes as it does with the addition of Na₂S. |
33.A Electrolytic Cells, Part A: Electrolysis of Aqueous Salt Solutions 电解池,A 部分:盐溶液的电解
请注意,在电解池中,阴极Cathode为 “-”,但在原电池中为 “+”;
在电解池中,阳极Anode为 “+”,但在原电池中为 “-”。
cathode⬇️阴极永远发生还原⬇️接收电子e-,anode⬆️阳极永远发生氧化⬆️给出电子e-;
在电解池中,阴极Cathode是负极-,阳极Anode是正极+;
在原电池中,阴极Cathode是正极+,阳极Anode是负极-。
Step #1: Set up the electrolysis apparatus 步骤#1:设置电解装置
The dc power supply can be a 9-V transistor battery.3Several drops of universal indicator can be added to the solution in both chambers to detect pHchanges
直流电源可以是一个9伏的晶体管电池。在两个腔室中的溶液中可以加入几滴通用指示剂,以检测pH变化。
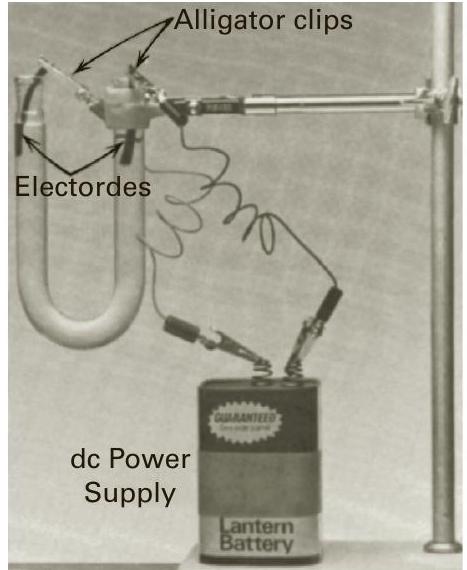
FIG. 33.3 Electrolysis apparatus
图 33.3 电解装置
Table 33.1 Electrolytic Cells for Study
| Solution No. | Solution* | Electrodes (Cathode and Anode) |
|---|---|---|
| 1 | Carbon (graphite) | |
| 2 | Carbon (graphite) | |
| 3 | Carbon (graphite) | |
| 5 | Carbon (graphite), Cu(s) |
表 33.1 用于研究的电解池
| 溶液编号 | 溶液* | 电极 (阴极和 阳极 ) |
|---|---|---|
| 1 | 碳 ( 石墨 ) | |
| 2 | 碳 ( 石墨 ) | |
| 3 | 碳 ( 石墨 ) | |
| 5 | C(gr), Cu(s) |
We will be doing trials with Solutions #1, #2, and #3 as described in Table 33.1. Skip Solution #4. Solution #5 will be performed as described below. Prepared solutions will be provided to you in lab.
For Solution #5, you will use solution in the U-tube. For this trial only, you will be using a piece of copper wire (cathode) and a carbon electrode (anode). Attach the wires to the battery and insert the electrodes into the zinc solution. Record any observations.
我们将按照表 33.1 中的描述,对溶液 #1、#2 和 #3 进行试验。溶液 #5 将按照以下描述进行。制备好的溶液将在实验室中提供给您。
对于溶液 #5,您将在 U 形管中使用 溶液。仅对于此试验溶液** #5**,您将使用一段铜线(阴极)和一个碳电极(阳极)。
Obtain a glass U-tube, two carbon electrodes and a battery from the front bench. The carbon electrodes are very fragile so be careful not to drop them and handle them carefully when cleaning and using them.
从前面的工作台上获取一个玻璃 U 形管、两个碳电极和一个电池。碳电极非常脆弱,因此请小心不要掉落它们,并在清洁和使用它们时小心处理它们。
Securely clamp the U-tube under the canopy hood. Place a piece of white paper or a Kim-Wipe underneath the U-tube to better see if any colors form in solution.
将 U 形管牢固地夹在通风橱下。在 U 形管下方放置一张白纸或 Kim-Wipe,以便更好地查看溶液中是否形成任何颜色。
Set up the electrolysis apparatus.
Connect two wireleads (different colors) attached to alligator clips to a direct current (dc) power supply.
Clean and mount the glass U-tube on a ringstand (see Figure 33.3).
Connect the alligator clips to the corresponding electrodes, listed in Table 33.1.
设置电解装置。
将连接到鳄鱼夹的两根导线(不同颜色)连接到直流电源。
清洁并将玻璃U 型管安装在环形支架上(参见图 33.3)。
将鳄鱼夹连接到表 33.1 中列出的相应电极。
Step #2: Electrolyze the solutions 步骤#2:电解溶液
Fill the U-tube three-fourths full with Solution 1 from Table 33.1. Insert the corresponding electrodes into the solution and electrolyze for minutes. During the electrolysis, watch for any evidence of a reaction in the anode and cathodechambers.
用表 33.1 中的溶液 1 将 U 型管注满四分之三。 将相应的电极插入溶液中并电解约 5 分钟。 在电解过程中,观察阳极和阴极腔室中反应的任何迹象。
1
Does the pH of the solution change at each electrode? Test each chamber with litmus or pH paper. Compare the color with a pH test on the original solution.
每个电极上溶液的 pH 值是否发生变化? 用石蕊或 pH 试纸测试每个腔室。 将颜色与原始溶液的 pH 测试进行比较。
2
Is a gas evolved at either or both electrodes? Look closely.
是否在任一或两个电极上释放出气体? 仔细看看。
3
Look closely at each electrode. Is a metal depositing on the electrode or is the metalelectrode slowly disappearing?
仔细观察每个电极。 金属是否沉积在电极上,或者金属电极是否正在慢慢消失?
4
For Solution #5, you will use solution in the U-tube. For this trial only, you will be using a piece of copper wire (cathode) and a carbon electrode (anode). Attach the wires to the battery and insert the electrodes into the zinc solution. Record any observations.
对于溶液 #5,您将在 U 形管中使用 溶液。仅对于此试验,您将使用一段铜线(阴极)和一个碳电极(阳极)。将电线连接到电池,然后将电极插入锌溶液Zn(NO₃)₂中。记录任何观察结果。
When finished, make sure that the contents of the U-tube get placed into the liquid waste container.
完成后,确保将 U 形管的内容物放入液体废物容器中。
When finished, return the equipment to the front bench.
完成后,将设备返回到前面的工作台。
Step #3: Account for your observations 步骤#3:说明您的观察结果
Make sure that you correctly record the pH readings and which electrode is which. Check that your observations seem reasonable. If unsure, ask your TA or Instructor.
确保您正确记录 pH 读数以及哪个电极是哪个。检查您的观察结果是否合理。如果不确定,请咨询您的 助教或导师。
You can write out the reactions for the lab report.
您可以为实验报告写出反应。
| Solution | Electrodes | Litmus Test | Gas Evolved? | Balanced Equations for Reactions |
|---|---|---|---|---|
| NaCl | C(gr) | Anode: | ||
| Cathode: | ||||
| Cell: | ||||
| NaBr | C(gr) | Anode: | ||
| Cathode: | ||||
| Cell: | ||||
| KI | C(gr) | Anode: | ||
| Cathode: | ||||
| Cell: | ||||
| Zn(NO₃)₂ | Cu(s), C(gr) | Anode: | ||
| Cathode: | ||||
| Cell: |
Waste Disposal 废物处理
Any remaining solutions need to be emptied into the liquid waste container under the canopy hood.
任何剩余的溶液都需要倒入通风橱下的液体废物容器中。
All glassware with chemical residue (test tubes, beakers, U-tube, etc.) should be rinsed 3 times with small aliquots of deionized water, per the EPA washing technique, and discarded into the liquid waste container. Additional washings can be done in the sink with water and if necessary, detergent.
所有带有化学残留物的玻璃器皿(试管、烧杯、U 形管等)应按照 EPA 清洗技术,用少量去离子水冲洗 3 次,然后丢弃到液体废物容器中。
可以在水槽中使用水和必要的洗涤剂进行额外的清洗。
Plastic pipettes should be placed in the plastic waste container found at the front of the room.
塑料吸管应放在房间前面的塑料废物容器中。
Used salt bridges should be placed in the solid waste container at the front of the lab.
用过的盐桥应放在实验室前面的固体废物容器中。
Don't forget to submit your prelab for Exp #7 on Lab Archives before you leave the lab.
别忘了在离开实验室之前,在 Lab Archives 上提交 Exp #7 的预实验报告。
Lab Report #7 Details 实验报告#7 详情
This is a notebook report. The prompts you need to answer will be provided to you in lab. They can also be found within the assignment on the UN1500 site. You will upload your completed notebookdiscussion to Courseworks.
这是一个笔记本报告。你需要回答的问题将在实验室提供给你。它们也可以在UN1500网站上的作业中找到。你将把完成的笔记本讨论上传到Courseworks。
Calculations for Report #7: 报告#7的计算:
All relevant data and results should be reported on the ReportSheets (Beran pages ). A hand-written calculation should be shown for every type of calculation. The calculations & completed ReportSheets will be submitted to your TA at the start of the next labperiod.
所有相关的数据和结果应该在报告表格(Beran pages )上报告。每种计算都应显示手写计算。计算和完成的报告表格将在下一个实验室期间开始时提交给你的助教。
32.A 伽伐尼电池;能斯特方程,A部分:几种氧化还原电对的还原电势Galvanic Cells; the Nernst Equation, Part A: Reduction Potentials of Several Redox Couples
For #1 and #2, complete as outlined in the labmanual.
对于#1和#2,按照实验手册中的概述完成。
For #3, there may be a typo in your manual. The statement should read as follows: "Compare the sum of the and cellpotentials with the Cu-Mg cellpotential. Explain." You should focus on why the values are similar, not why they are different. They are different due to experimental error.
对于#3,你的手册中可能存在排版错误。该声明应如下所示:“将和电池电位的总和与Cu-Mg电池电位进行比较。解释。” 你应该关注为什么这些值相似,而不是为什么它们不同。由于实验误差,它们是不同的。
For #4, complete as outlined in the labmanual. You should focus on why the values are similar, not why they are different. They are different due to experimental error.
对于#4,按照实验手册中的概述完成。 你应该关注为什么这些值相似,而不是为什么它们不同。 由于实验误差,它们是不同的。
For #5, complete as outlined in the labmanual. We did not have an unknown so skip that row.
对于#5,按照实验手册中的概述完成。 我们没有未知的,所以跳过那一行。
Disregard "#6" since we did not complete that portion of the experiment.
忽略“#6”,因为我们没有完成实验的那个部分。
There is no erroranalysis for this portion of the labreport, but pay attention to significant figures. Reductionpotentials given to you in the manual or in the lecture should be treated as constants.
此实验报告的部分没有误差分析,但请注意有效数字。 在手册或讲座中给你的还原电位应被视为常数。
32.B 伽伐尼电池;能斯特方程,B部分:浓度变化对电池电位的影响 Galvanic Cells; the Nernst Equation, Part B: Effect of Concentration Changes on Cell Potential
Complete as outlined on the ReportSheet.
按照报告表格上的概述完成。
33.A 电解池,A部分 Electrolytic Cells, Part A
Complete the ReportSheet in the labmanual. Refer to the lectureslides or Beran manual for relevant half reactions.
完成实验手册中的报告表格。 有关相关的半反应,请参阅讲座幻灯片或Beran手册。